News Center
─ Specialized manufacturer • since 2005 ─
NEWS CENTER
A batch of medical devices were unqualified, and the Food and Drug Administration ordered a "recall for rectification"!
- Categories:Industry News
- Author:
- Origin:
- Time of issue:2022-01-07
- Views:0
(Summary description)Jiangsu Provincial Food and Drug Administration, Siyu Research Institute, Medical Device Dealers Alliance
A batch of medical devices were unqualified, and the Food and Drug Administration ordered a "recall for rectification"!
(Summary description)Jiangsu Provincial Food and Drug Administration, Siyu Research Institute, Medical Device Dealers Alliance
- Categories:Industry News
- Author:
- Origin:
- Time of issue:2022-01-07
- Views:0
Source: Jiangsu Provincial Food and Drug Administration, Siyu Research Institute, Medical Device Dealers Alliance
Sorting out: Union bacteria
On March 9, the Jiangsu Provincial Food and Drug Administration issued the "Notice on the Quality of Medical Devices and Drug Packaging Materials for Phase 2 of 2020." The supervision of medical devices and pharmaceutical packaging materials has been carried out.
A batch of medical devices were recalled
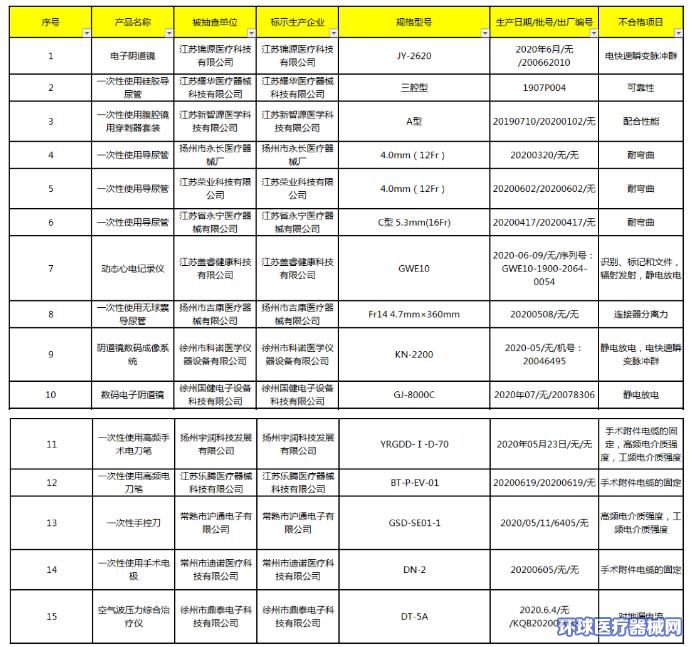
The notice pointed out: In order to strengthen the quality supervision of drugs and medical devices and ensure the effective use of products, in accordance with the annual sampling work plan of the Jiangsu Provincial Drug Administration, the Jiangsu Provincial Drug Administration organizes the production, operation and use of medical devices and drug packaging materials. Random inspections were conducted. Among them, no unqualified medical packaging materials were found, and 15 batches of medical devices were unqualified.
After sampling, it was found that the non-conforming medical devices mainly include electronic colposcopy, disposable silicone urinary catheter, disposable laparoscopic trocar kit, 3 batches of disposable urinary catheter, Holter recorder, one-time use Sexual use of non-balloon catheter, colposcopy digital imaging system, digital electronic colposcopy, disposable high-frequency surgical electrosurgical pen, disposable hand-controlled knife, disposable surgical electrode, air wave pressure comprehensive treatment instrument, disposable Use a high-frequency electrosurgical pen. Non-conforming items include electrical fast transient pulse group, reliability, coordination performance, etc. The details are as follows:
The Jiangsu Provincial Drug Administration stated that for the unqualified products found in the above-mentioned random inspections, the drug regulatory authorities at all levels in our province have urged companies to conduct risk assessments of related products, determine the recall level based on the severity of medical device defects, and take the initiative to recall the products. Disclosure of recall information; require companies to find out the reasons for product unqualified as soon as possible, formulate corrective measures and make corrective actions in place on schedule. at the same time. The relevant production, operation and use units of substandard products have been organized to investigate and deal with in accordance with the law.
Medical device recall system
On January 25, 2017, the ** Food and Drug Administration passed the "Medical Device Recall Management Measures." According to the different initiators of medical device recalls, medical device recalls are divided into active recalls and ordered recalls.
Active recall refers to the quality assessment of medical device products produced by medical device manufacturers in accordance with relevant requirements or product adverse events and other information. It is a legal obligation for manufacturers to actively recall defective medical devices. To order a recall, the Food and Drug Administration, after investigation and evaluation, believes that a medical device manufacturer should recall a defective medical device product without voluntarily recalling it, and orders the medical device manufacturer to implement a medical device recall. In practice, it is generally based on the initiative of the enterprise to recall, supplemented by the government department ordering the recall.
According to the severity of medical device defects, medical device recalls are divided into:
First-level recall: the use of the medical device may or has caused serious health hazards or even death; the second-level recall: the use of the medical device may or has caused temporary or reversible health hazards; the third-level recall: the use of the medical device may cause harm It is less likely to be recalled but still needs to be recalled.
The risk of medical devices is gradually reduced from the first to the third level. Therefore, if the medical device manufacturer makes a medical device recall decision, the response to the first-level call is within 1 day, the response to the second-level call is within 3 days, and the response to the third-level call within 7 days , Notify the relevant medical device business enterprise, user unit or user.
Active "recall" is a manifestation of responsibility
For medical companies that actively "recall" problematic products, we can understand that this is a responsible performance. However, the products in the above cases were randomly inspected by the China Food and Drug Administration and the companies were urged to carry out the recall. We can't beat them to death and say that these companies are irresponsible. They just control the quality of their products. At this stage, most domestic companies are not doing enough!
In recent years, with the gradual improvement of medical device reviews by local regulatory agencies, the number of medical device recalls has increased rapidly, and the word "recall" has gradually changed from a sensitive word to a common word and appeared in the public eye.
At this stage, it is not only that the supervision of medical devices has increased, but it is also more and more common for companies to take the initiative to find problems and recall them. This represents an improvement in the awareness and level of risk management in the entire domestic medical device industry. According to the article published by the "Siyu Research Institute", compared with the worries of the Chinese people, the western developed people's attitude towards the "recall" is completely different. In particular, medical institutions in the United States generally remain calm when faced with "medical device recalls" and even appreciate that companies are responsible for taking the initiative to recall products with potential risks. Many people said that voluntarily initiating the recall of defective medical devices or potentially dangerous products is a good performance for medical device manufacturers to ensure the use of public devices and fulfill their social responsibilities.
Perhaps it is precisely because the company's sense of responsibility is reflected behind the recall, its product sales have risen instead of falling. According to a report by Business Weekly on April 2, 2014, General Motors in the U.S. was deeply troubled by the uproar of vehicle recalls, but unexpectedly, the company's sales in the U.S. market in March increased by 4% year-on-year. It is also worth mentioning that none of the world's famous automakers such as Mercedes-Benz, BMW, and Volkswagen have ever implemented a recall. Compared with lies and cover-ups, the public tends to prefer companies that are willing to proactively admit their mistakes and correct them.
No company can guarantee that the products it manufactures are free of problems, not to mention medical devices! Any medical device has a certain risk of use, and the medical device approved for marketing is only a product with acceptable risks. The recall of defective medical devices is the need to promote the advancement of production technology and improve the competitiveness of enterprises. Recall is an important measure for the supervision of medical devices after they are on the market, which can significantly eliminate or reduce the risks and hazards of defective medical devices to ensure medical care. Strengthening the strength of unannounced medical device inspections and establishing a sound medical device recall system are effective means to control medical device risks, protect users' **, and improve product design.
Scan the QR code to read on your phone
LATEST NEWS

Time of issue : 2023-09-29

Time of issue : 2023-06-16
Let's share with you the extraordinary moments with you.
Let's share with you the extraordinary moments with you.

Time of issue : 2023-04-13

Time of issue : 2023-03-09
Keewell Medical Technology Co., Ltd.

Add: Xiananyi Industry Zone, Pingzhou,Nanhai District, Foshan City,Guangdong Province 528251, P.R.China

Tel: +86-757-86260855

Email: marketing@keewell.cn
Copyright 2021@ Keewell Medical Technology Co., Ltd. 粤ICP备16061208号 www.300.cn SEO TAGS


 CHINESE
CHINESE ENGLISH
ENGLISH  ESPAÑOL
ESPAÑOL




